Questions and Answers on CoViD19
On this page, we answer common questions related to the Novel Coronavirus disease (CoViD-19). We rely only on reputed scientific and factual sources from around the world while answering.
The resources here are based on the availability of current scientific evidence, and will be updated as new data emerges.
[Dates in parentheses indicate the date answers were last updated]
- [28/05/2021] Q32. What do we know about the B.1.617 variant?
- [28/05/2021] Q31. Can infection happen in vaccinated individuals?
- [28/05/2021] Q30. Will vaccination alter the mortality rate of the current wave?
- [28/05/2021] Q16. What is convalescent plasma therapy, and can it be used to treat COVID-19?
- [28/05/2021] Q15. Can someone be reinfected after they have recovered from COVID-19?
- [28/05/2021] Q12. Do chloroquine and hydroxychloroquine help in treating COVID-19?
- [28/05/2021] Q11. Can children and young people get sick with coronavirus ?
- Q29. What makes some COVID-19 cases fatal?
- Q28. How can pregnant women, senior citizens, or people with compromised immune systems protect themselves from COVID-19?
- Q27. What is the role of an asymptomatic or presymptomatic carrier in the spread of COVID-19?
- Q26. Why do some COVID-19 patients suffer from blood clots and strokes?
- Q25. I see “viral load” and “viral shedding” in news about COVID-19. What are these, and what do they tell us?
- Q24. Are there different strains of the novel coronavirus, and will a vaccine work against all of them?
- Q23. How do COVID-19 contact tracing apps work?
- Q22. How do homemade masks help in preventing COVID-19 infection?
- Q21. What are the stages of the COVID-19 pandemic?
- Q20. Once someone inhales contaminated droplets, how does the novel coronavirus enter their cells?
- Q19. Is a second wave of the pandemic likely to occur?
- Q18. What is serological testing and how can it be used to detect COVID-19?
- Q17. What is herd immunity?
- Q14. How does our immune response work and can we “boost” it to protect against COVID-19?
- Q13. Can BCG vaccination protect me against COVID-19?
- Q10. What makes the novel coronavirus (SARS-CoV2) more dangerous than Flu and SARS-CoV1 viruses?
- Q09. How long can the novel coronavirus survive in air and on various surfaces?
- Q08. What are the symptoms of COVID-19?
- Q07. Will the summer temperature and humidity affect the novel coronavirus in India?
- Q06. How do droplets spread Covid-19?
- Q05. How long does it take before one shows symptoms of Covid-19?
- Q04. How does physical distancing help reduce the spread of novel coronavirus?
- Q03. I have heard that there are many types of coronaviruses. Can they not infect humans?
- Q02. Why does it take a long time to make a vaccine against the novel coronavirus? Can the process be made faster?
- Q01. What exactly is a testing kit? Why is it difficult to make one for COVID-19, and why do the results take time?
Q32. What do we know about the B.1.617 variant?
Q31. Can infection happen in vaccinated individuals?
Q30. Will vaccination alter the mortality rate of the current wave?
Q29. What makes some COVID-19 cases fatal?
Last update: 15 June 2020
Case fatality rate/ratio (CFR), is the ratio of the deaths occurring from a particular cause, to the total number of cases from the same cause. If 100 people were diagnosed with the disease and 10 of them die, the CFR is 10/100, or 10%.
Studies show that increased probability of death in COVID-19 patients is associated with older age. With age, the suppressed immune system reduces the ability to fight viral infections. This could lead to organ failure, and higher propensity for blood clot formation, e.g., see reports by Walker et al., 2020 and Zhou et al., 2020.
Researchers have identified additional factors which increase the fatality risk, such as (1) being male, (2) having an underlying cardiovascular (related to heart function and blood flow) or cerebrovascular (related to blood flow in the brain) disease, diabetes, hypertension or high levels of cardiac muscle protein i.e. troponin I (indicative of heart injury), (3) having chronic obstructive pulmonary disease or cancer, (4) low counts of cytotoxic T cells (i.e. fewer immune cells that can kill virus-infected cells) and (5) high corticosteroid use. The precise reason for why these factors increase the CFR for COVID-19 remains unknown.
- Why does COVID-19 disproportionately affect older people?
- OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients.
- Clinical Course and Risk Factors for Mortality of Inpatients With COVID-19
- Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China
- Predictors of Mortality for Patients with COVID-19 Pneumonia Caused by SARS-CoV-2: A Prospective Cohort Study
- Covid-19: risk factors for severe disease and death
- Risk Factors for Severity and Mortality in Adult COVID-19 Inpatients in Wuhan
Q28. How can pregnant women, senior citizens, or people with compromised immune systems protect themselves from COVID-19?
Last update: 15 June 2020
Senior citizens, pregnant women and those with a weaker immune system (e.g. patients undergoing chemotherapy or with any other illness) must strictly follow measures of physical distancing, go out only when absolutely necessary, wear masks while outside, and practice hand hygiene.
A. Pregnant women
So far, there is no conclusive evidence for transmission of COVID-19 from an infected woman to the fetus in the uterus (see this analysis, and a review). However, the immune response to COVID-19 could potentially affect the developing fetus (Liu et al., 2020). At the time of delivery, the mother may transmit the infection to the newborn due to close physical contact or through care-givers. As we are just beginning to understand the novel coronavirus, it is difficult to predict long-term effects on the newborn. Hence, pregnant women need to be extra cautious to reduce their risk of contracting COVID-19.
In addition to the standard measures above and taking all the recommended vaccines as per schedule, the Centres for Disease Control and Indian Council of Medical Research (ICMR) recommend reducing the number of hospital visits during pregnancy, in consultation with the maternal care provider. Hospital travel should be limited to either private vehicles or hospital ambulances.
B. Senior citizens or people with compromised immune systems
Senior citizens and people with compromised immunity such as cancer patients are at a higher risk from COVID-19 as shown by several studies, e.g. reported by Leung 2020 and Dai et al., 2020 . Considering the severity of the disease and higher mortality rate among senior citizens and those with underlying comorbid conditions, transmission can be reduced by following strict precautionary measures as follows.
- Stay at home unless absolutely essential, and avoid visitors at home. Keep physical distance from others.
- Take medications for any underlying health conditions exactly as prescribed.
- Postpone surgeries such as cataract or other interventions that are not urgent.
More information can be found in the advisory issued by MoHFW and guidance by CDC.
References
- An Analysis of 38 Pregnant Women with COVID-19, Their Newborn Infants, and Maternal-Fetal Transmission of SARS-CoV-2: Maternal Coronavirus Infections and Pregnancy Outcomes
- Vertical Transmission of Severe Acute Respiratory Syndrome Coronavirus 2: A Systematic Review
- Why are pregnant women susceptible to COVID-19? An immunological viewpoint
- If You Are Pregnant, Breastfeeding, or Caring for Young Children
- Guidance for management of pregnant women in COVID-19 pandemic
- Risk factors for predicting mortality in elderly patients with COVID-19: A review of clinical data in China
- Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak
- Health advisory for elderly population of India during COVID-19
- COVID-19 guidance for older adults
Q27. What is the role of an asymptomatic or presymptomatic carrier in the spread of COVID-19? (Reader Question)
Last update: 15 June 2020
Once the novel coronavirus infects a person, it takes about 5 days to develop symptoms (but this period can vary from 2 to 15 days). This phase is called the ‘incubation period’; if tested positive during this period, the patient is classified as “presymptomatic”. A subset of infected people can carry the virus in their body, but never develop any symptoms of the disease. They appear healthy but can still spread the virus to other people. These are termed as “asymptomatic carriers”. In case of COVID-19, 5-80% of the infections are asymptomatic (Melissa M. et al 2020, Michael 2020, Michael 2020, Oran et al 2020, Xi He et al 2020). The amount of virus in both asymptomatic and presymptomatic cases can be as high as that in symptomatic patients. Both asymptomatic and presymptomatic people can transmit the virus to others. This is thought to explain the rapid spread of COVID-19, and seems to be one of the major differences between COVID-19 and the previous coronavirus infections (SARS and MERS) that could be controlled relatively easily.
If there are no symptoms like coughing or sneezing, how can someone transmit the disease?
The novel coronavirus is transmitted primarily through droplets released while sneezing or coughing. However, small droplets are also generated when we talk or exhale air, and these may also carry virus particles. A recent study found out that even loud speech generates more than 1000 droplets per minute, which can remain air-borne for about 8 min. Although most large droplets eventually settle down within 1 m, smaller droplets or even larger droplets in some cases can travel 7-8 m. The distance travelled by droplets and their role in transmitting the virus is being investigated. Article in Lancet has supported the role of physical distancing of 1 m or more in reducing the risk of transmission. Another mode of transfer is through direct contact such as handshakes, or through shared objects (‘fomites’ like utensils and towels). A recent study showed that undocumented infections may account for as high as 55% of total transmission.
Why are some people asymptomatic but others show symptoms?
The symptoms and severity of an infectious disease is a complex outcome of many factors, such as features of both the pathogen and the host (e.g. their respective genetic makeup, the host’s overall health and immune function), and the interaction between the host and pathogen. In the case of COVID-19, we do not have a clear understanding of why some people develop COVID-19 symptoms while others do not.
How can we stay safe from asymptomatic carriers?
Follow physical distancing, and personal and public hygiene. The best way is to assume that you yourself or other people are already infected with the novel coronavirus. Accordingly, avoid close contact with others, even if you/they seem healthy.
References and more information:
- The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application
- Evidence Supporting Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 While Presymptomatic or Asymptomatic
- Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility
- Covid-19: four fifths of cases are asymptomatic, China figures indicate
- Prevalence of Asymptomatic SARS-CoV-2 Infection: A Narrative Review: Annals of Internal Medicine
- Evidence Supporting Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 While Presymptomatic or Asymptomatic
- Temporal dynamics in viral shedding and transmissibility of COVID-19
- SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients
- Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19
- Violent expiratory events: on coughing and sneezing
- Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2)
- The coronavirus pandemic and aerosols: Does COVID-19 transmit via expiratory particles?
- Reducing transmission of SARS-CoV-2
- Turbulent Gas Clouds and Respiratory Pathogen Emissions
- Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community
- The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission
- WHO: Coronavirus disease (COVID-19) advice for the public
Q26. Why do some COVID-19 patients suffer from blood clots and strokes?
Last update: 15 June 2020
COVID-19 positive cases span a range of outcomes, from about 80% being asymptomatic to about 5% becoming critically ill and exhibiting Acute Respiratory Distress Syndrome (ARDS), according to data from China. Some patients also exhibit non-respiratory symptoms such as acute liver and heart injury, kidney failure, or diarrhoea (as referenced here, here, and here).
Upon gaining entry through the ear, nose, or mouth, the novel coronavirus binds to the angiotensin converting enzyme-2 (ACE-2) receptor (a protein) on human cells in the nose, bronchi (corridors through which air passes), lungs, heart, esophagus, kidney, stomach, bladder, and ileum. Initially, the novel coronavirus reproduces in the cells lining the inside of the upper respiratory tract (nasal cavity and pharynx), with further multiplication in the lower respiratory tract and cells lining the gastrointestinal system (stomach and intestines), as reported here.
During the COVID-19 epidemic, the severe deterioration of some patients has been closely related to the cytokine storm in their bodies. Cytokines are very small proteins that are produced by many types of cells, including immune cells. They help cells to communicate with each other. In the immune system, cytokines regulate the balance between antibody based immune response and cellular immune responses. In a cytokine storm, levels of some cytokines soar far beyond what is needed, causing immune cells to attack healthy tissues. As a result, in some patients blood vessels leak and blood pressure drops. To compensate, blood clots form; but they can lead to severe organ failure, as discussed in this review. Strokes are often caused by blood clots that break free and travel to blood vessels in the brain. Thus, doctors link COVID-19 to potentially deadly blood clots (as shown here) and strokes (discussed in strokes and blood clots in COVID-19 patients).
During COVID-19 related cytokine storms inflammatory cytokines such as IL-1beta, IFN-γ, IP-10, and monocyte chemoattractant protein 1 (MCP-1) that activate the T-helper type 1 (Th1) cell response against intracellular parasites such as viruses (see this report) are elevated. According to another review, the severity of COVID-19 is related to levels of two specific cytokines (IL-2R and IL-6) in the blood of patients.
The pathogenesis of COVID-19, and disorders associated with infection by the novel coronavirus, is depicted here, here and here.
References:
- Viruses | Free Full-Text | Virology, Epidemiology, Pathogenesis, and Control of COVID-19
- Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China
- Kidney impairment is associated with in-hospital death of COVID-19 patients
- Clinical Characteristics of Patients With 2019 Novel Coronavirus (2019-nCoV)–Infected Pneumonia in Wuhan, China
- Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection
- Evidence for Gastrointestinal Infection of SARS-CoV-2.
- Definition of cytokine storm – NCI Dictionary of Cancer Terms
- How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from brain to toes
- The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19
- https://www.ncbi.nlm.nih.gov/pubmed/31986264
- COVID-19 cytokine storm: the interplay between inflammation and coagulation
- Strokes And Blood Clots Seen In COVID-19 Patients : Shots – Health News
- Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young | NEJM
- Viruses | Free Full-Text | Virology, Epidemiology, Pathogenesis, and Control of COVID-19 | HTML
- PubMed Central, Fig. 2: J Autoimmun. 2020 May; 109: 102433. Published online 2020 Feb 26. doi: 10.1016/j.jaut.2020.102433
- Appreciating potential complications of COVID-19
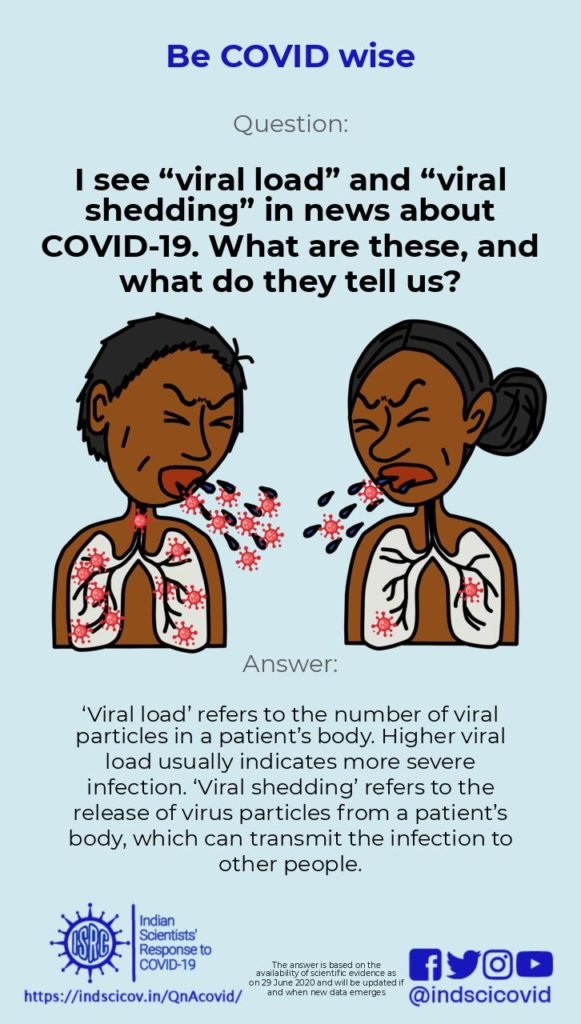
Q25. I see “viral load” and “viral shedding” in news about COVID-19. What are these, and what do they tell us?
Last update: 15 June 2020
Viral Load: Also known as ‘viral burden’, it is defined as the number of viral particles in a fixed amount of body fluid. Since COVID-19 is a respiratory disease (caused by the novel coronavirus), its viral load is measured in nasal/oral droplets or fluids. The viral load gives an indication of the extent of infection (severity). Depending on the diagnostic method used, the viral load can be calculated from the nucleic acid (RNA) copy number or from the viral antigens present in a patient. For COVID-19, the primary diagnostic test uses a technique called RT-PCR to detect and estimate viral loads in nose and throat swabs. The viral load decreases as a person enters the recovery stage of COVID-19.
Viral Shedding: Viral shedding is a measure of the transmissibility of the infection. Once a virus gets inside a cell, it hijacks that cell and uses it to make more viruses. These are released into the body and infect new cells, leading to an increase in the viral load in the host. The new virus particles are also released outside the body, through respiratory droplets and aerosols. Such “shedding” of the novel coronavirus is known to occur even before an infected person begins to show symptoms. Therefore, everyone is advised to wear a face mask to prevent transmission in case of a pre-symptomatic or asymptomatic infection.
References and more information:
- Detection of SARS-CoV-2 in Different Types of Clinical Specimens
- Viral dynamics in mild and severe cases of COVID-19
- Diagnosing COVID-19: The Disease and Tools for Detection
- FDA Statement: Coronavirus (COVID-19) Update: FDA Authorizes First Antigen Test to Help in the Rapid Detection of the Virus that Causes COVID-19 in Patients
- Quantitative Detection and Viral Load Analysis of SARS-CoV-2 in Infected Patients
- Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens for COVID-19
- SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor
- Respiratory virus shedding in exhaled breath and efficacy of face masks
- Temporal dynamics in viral shedding and transmissibility of COVID-19
Q24. Are there different strains of the novel coronavirus, and will a vaccine work against all of them?
Last update: 15 June 2020
All organisms acquire mutations (changes in the genetic code) while replicating. Viruses have a high mutation rate, especially those with RNA as their genetic material. However, coronaviruses (including SARS-CoV-2) are an exception, partly due to their proofreading mechanism that corrects most mutations.
A mutation does not always lead to a new virus strain. A ‘new strain’ differs from its ancestor in significant ways. It might vary in how easily it spreads, its ability to cause disease, whether it is recognized differently by the immune system, or how differently it responds to medications. Most mutations do not affect these properties and are silent. Hence, without information on the effects of specific mutations, there is insufficient information to classify the virus as a new ‘strain’. Currently, claims regarding diversification of SARS-CoV-2 into various strains due to mutations are not supported by conclusive evidence.
Will the different sequences of the circulating novel coronavirus affect the efficacy of vaccines which are currently under development? Antibodies, which the body produces in response to a vaccine (or the virus), work by binding to specific regions of a virus, called antigens. If viral mutations alter the antigen, they can make a vaccine less effective against the virus. However, currently we do not know whether the observed mutations in the virus affect its antigenic properties. Hence, it is too early to speculate about the possible effects on vaccines. But scientists think that the efficacy of the vaccines towards the mutating virus may not be a problem in the immediate future, owing to the slow mutation rate of the novel coronavirus.
More research is required to know if different strains of the novel coronavirus exist and if a vaccine will work properly against all of them.
References:
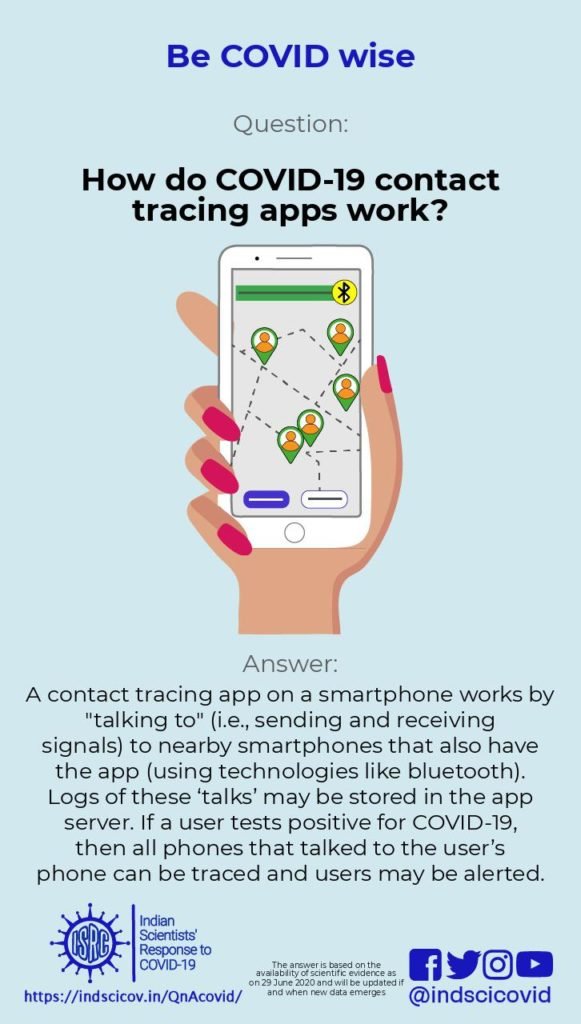
Q23. How do COVID-19 contact tracing apps work?
Last update: 15 June 2020
A contact-tracing app is a smartphone app that helps locate other smartphones with the same app that have been physically close to each other. Such apps are being used in some countries, including India, to help contain the spread of COVID-19 [BBC]. These apps have been largely inspired by the success of the Trace Together app used in South Korea [BBVA]. However, the success story in Korea is based not only on the smartphone app, but also on implementing other strategies such as large scale testing, observing physical distancing norms, wearing masks, and extensive contact tracing [National Geographic].
The apps require users to give information about their home address, gender, and age; and in some cases, past ailments. Users are also given a unique user id attached to their smartphone. Users sign an agreement that if they test positive for COVID-19, the government can request data on their contacts and movements from their service provider (e.g. Jio, Airtel, BSNL). Whenever they go out of their home, users need to turn on their phone’s bluetooth, so that information about their movements can be collected.
Suppose X has this app and is diagnosed with COVID-19 today. Everyone who has been in contact with X in the past two weeks (the incubation period of the virus) is contacted. They are asked to self-quarantine because there is a chance that X may have transmitted the infection, and they may also unknowingly give it to others.
How do these apps trace people who have been in contact with X?
When two people (X and Y) who have both downloaded these apps are within 2-3 metres of each other, their cell phones exchange messages using bluetooth. The user id of X is encrypted using a secret key unique to Y, and is stored on Y’s phone. Similarly, the user id of Y is encrypted using a key unique to X, and is stored on X’s phone. Encryption means a user id is transformed using a secret key, in such a way that it is computationally difficult to recover the user id from the transformed version, unless you have the key. The encrypted user ids are stored for about two weeks. If X develops COVID-19, all encrypted user ids stored on X’s phone from the previous two weeks are collected by government agencies. They decrypt (unscramble) the user ids stored on X’s phone (using X’s secret key, which they have access to), so that all users whose phones were in close proximity to X’s phone can be traced, contacted and warned that they may have picked up an infection. The unscrambling of encrypted ids on X’s phone can only be done by government agencies. Alternately, the app stores the messages exchanged between the two phones, on both the phones, for two weeks. If X falls ill with COVID-19, all messages stored on X’s phone are downloaded onto a central server. Every day the app on Y’s phone contacts this server to see if there is a message on Y’s phone that is also there on the server. If such a message is found, Y gets alerted about having been physically close to someone who has the disease. See the article by Nicky Case where this is explained using cartoons.
If these apps are useful, why are people concerned about using them?
An issue with such apps is privacy. Is the collected data stored on the phone, or on a centralized server? Most people would be happy if it is stored on their phone, since their data is safe with them. If it is stored on a centralized server, is the data safe? Who has access to it? Is the data encrypted before storing, so that anonymity of users is ensured? How does one know that the data will only be used for COVID-19 tracking? Shouldn’t the use of such an app be voluntary? These articles in Nature and National Herald discuss these issues at length.
What steps are being taken to address these issues?
Software leaders such as Google and Apple, and several researchers in Europe and in the US, are working on developing an app which meets stringent requirements for anonymity, privacy and security, and also scales to a large number of users. Using ideas from cryptography, researchers have proposed solutions which do not require a central server to store the data. See this MIT article for details on the issues involved, and the solutions being proposed.
References and more information:
- Nature: COVID-19 digital apps need due diligence.
- BBVA: How do COVID-19 tracing apps work and what data do they use?
- BBC: Source code behind contact tracing apps.
- National Herald: Is privacy a myth?
- Nicky Case, Protecting Lives & Liberty.
- MIT: A flood of coronavirus apps are tracking us.
- National Geographic, How South Korea prevented a coronavirus disaster, May 2020.
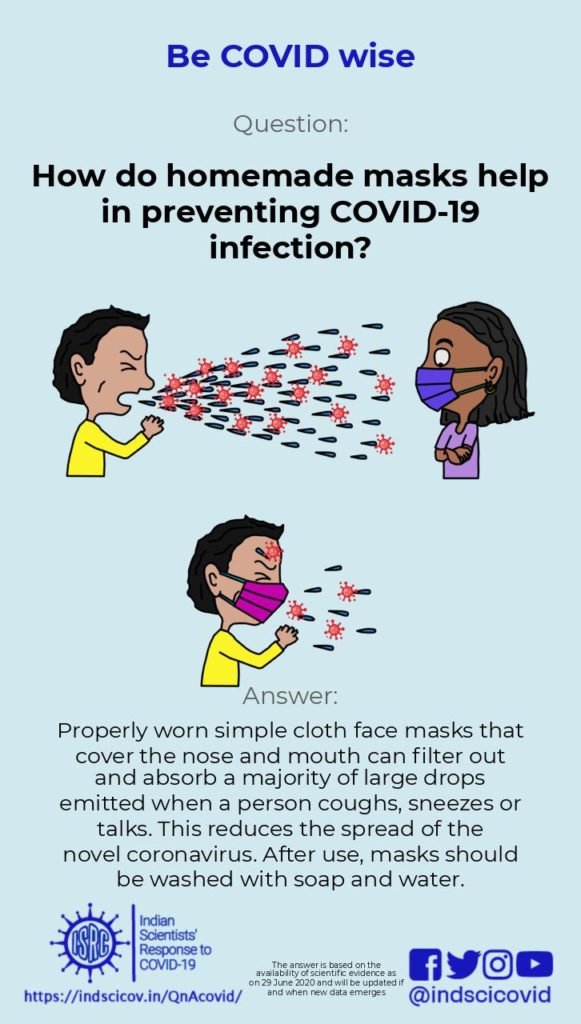
Q22. How do homemade masks help in preventing COVID-19 infection?
Last update: 15 June 2020
Advisories from the office of the Principal Scientific Advisor to the GoI and from the CDC recommend wearing homemade masks for controlling the spread of Covid-19. Transmission of the novel coronavirus is thought to happen primarily through droplets emitted when an infected person coughs, sneezes or speaks. Large droplets, typically larger than 10 microns in size, settle quickly to the ground due to gravity, typically within a distance of 1-2 m of the person. Smaller droplets (less than 5 microns) form “aerosols” that can stay suspended in the air for several minutes and propagate as far as 7-8 m. Currently, transmission of Covid-19 is believed to happen via large droplets rather than smaller aerosols. However, this is debated, and recent reports have suggested the possibility of aerosol transmission of the novel coronavirus (see recent publications in JAMA and Nature).
Health workers and caregivers who are directly in contact with infected patients are advised to use fitted N95 respirators that are highly effective in filtering out small particles. However, due to increased demand during the pandemic, N95 respirators and surgical masks are in short supply. Therefore, the general public is advised to use homemade face masks or face covers. Some people infected with the novel coronavirus do not show any symptoms but are capable of transmitting the disease. Therefore, it is suggested that the use of face masks can play an important role in controlling Covid-19. Wearing a facemask changes the nature of the turbulent expulsion of air during sneezing or coughing. Also, simple fabric face masks filter out and absorb a majority of the emitted large drops, preventing release of the virus into the environment. Recent research has suggested that face masks from a combination of fabrics (such as cotton, silk, synthetic materials) can provide improved filtration. However a standard homemade 2-layer cotton cloth mask with strings to tie at the back of the head will provide protection, especially when used in addition to physical distancing. ISRC has videos on making masks at home and how to use masks correctly.
It must be emphasized that wearing masks alone is insufficient to control virus transmission, and needs to be supplemented by rigorous physical distancing and hand wash hygiene to prevent the spread of Covid-19. In addition, when you wear a mask try to ensure that it fits well with no gaps. When you take it off, do not touch its outside surface; disinfect the mask right away (by boiling or washing with soap and water), and wash your hands with soap and water.
References and more information:
- PSA manual on facemasks
- CDC advisory on facemasks
- WHO note on transmission of Covid-19 through large droplets
- JAMA paper highlighting possible importance of aerosols
- Nature paper highlighting possible importance of aerosols
- Imaging the propagation of cough through a facemask
- Combinations of fabrics for improved filtration by facemasks
Q21. What are the stages of the COVID-19 pandemic?
Last update: 15 June 2020
A country (or location) may go through four different levels of COVID-19 transmission, as classified by WHO:
i. No cases reported
ii. Sporadic cases
iii. Cluster of cases
iv. Community Transmission
1. No cases reported: In India, the first case of COVID-19 was reported on 30th January, 2020. So, until 30/01/2020, India was at this level.
2. Sporadic cases: At this level, everyone detected with COVID-19 must have a travel history from an affected country, within the incubation period of the virus (upto 14 days for COVID-19).
3. Clusters of cases (also known as Local Transmission stage): At this stage, the transmission of the infection can be traced. People who test positive either travelled themselves to an affected region, or someone in their close contact group (such as family members) have travel history within the incubation period of the virus.
4. Community transmission: A country is said to enter the Community Transmission stage if at least one of these criteria is fulfilled: (a) a large number of confirmed cases cannot be traced through chains of transmission, or (b) a very high number of positive cases are found in random samples collected from patients with respiratory illness (known as sentinel samples). Countries like Italy, Spain, and the USA are at this stage.
Strategies to be followed at different stages, as suggested by WHO:
Clusters of cases: At this stage, the goal is to prevent from going to the community transmission stage. To achieve this, it is suggested that all cases should be rapidly found, isolated and treated; and all their contacts should be traced, quarantined, and supported (track-test-treat).
In addition, physical distancing and restrictions on domestic and international travel help in preventing the pandemic from progressing to the next stage.
Community Transmission: At this stage, it is difficult to stop the spread of the disease because of difficulty in isolating probable carriers. All public health measures including awareness campaigns about hand washing, physical distancing, and prevention should continue. In addition, testing laboratories should be prepared to handle a large number of samples. If testing capacity is limited, priority should be given to vulnerable populations and high-risk groups including healthcare workers. More details can be found here and here.
Note that the measures mentioned here at varying times in the local evolution of the COVID-19 pandemic are not set in stone. Countries are reviewing the situation regularly and taking a range of public health and social measures in different combinations.
References and more information:
- COVID-19 Situation Report prepared by WHO
- COVID-19 Strategy Update prepared by WHO
- Preparation for large scale community transmission: Guideline by WHO
- Laboratory testing strategy recommendations for COVID-19: Guideline by WHO
- Responding to Community Spreading: Guideline by WHO
- Operational considerations for case management of COVID-19 in health facility and community Interim guidance
Q20. Once someone inhales contaminated droplets, how does the novel coronavirus enter their cells?
Last update: 15 June 2020
The virus from respiratory droplets or surfaces enters our respiratory tract through the mouth, nose, or eyes. The genetic material of the novel coronavirus (RNA) is surrounded by a layer of protein, forming the nucleocapsid. This, in turn, is enveloped by a layer of modified fats called phospholipids. The outer envelope has proteins called S (for spike) glycoproteins studded in it. The first step of viral entry is the binding of the S protein to its receptor on the human cell. A part of the S protein called the fusion peptide is normally buried inside the S protein. Once bound to its receptor on the host cell (ACE 2), the S protein undergoes subtle changes in shape. This causes the fusion peptide to be brought to the surface, where it can get embedded into the host cell’s membrane. Another part of the S protein called the transmembrane domain is also brought near the fusion peptide, allowing the viral membrane to fuse with the host cell’s membrane. Modifications (mutations) to the S protein enable different coronaviruses to infect different host animals via different receptors.
Note that the virus cannot enter through intact skin, because skin cells lack the ACE 2 receptor. However, the novel coronavirus can enter any human cell that has the ACE2 receptor protein in the membrane (primary targets being nasal and lung tissue because the virus spreads primarily through droplets). Different tissues have different amounts of the ACE2 receptor in their cells. Although the virus primarily spreads by droplets and infects the lung and respiratory tract, in severe cases it can potentially infect many other tissues, such as the heart vessels, liver and kidney. It is now widely accepted that the virus spreads from person to person by reproducing and producing viral particles from cells in the oral cavity, which show high expression of the ACE2 receptor. However, lung tissues of diseased vs. healthy patients do not have significantly different amounts of the ACE2 receptor.
Although no direct information is available for the novel coronavirus, studies in a closely related coronavirus show that the virus can also enter cells in a completely different manner. Upon infection, our immune system generates antibodies against viral proteins, including the S protein. Sometimes, binding to the antibody can also change the shape of the S protein and activate it (as described above with the ACE2 protein). Meanwhile, cells that have receptors for the antibody can bind to this antibody-virus complex, allowing the virus to piggyback with the antibody and enter those cells.
References and more information
- Mechanisms of Coronavirus Cell Entry Mediated by the Viral Spike Protein
- COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses
- Molecular Mechanism for Antibody-Dependent Enhancement of Coronavirus Entry
- Structures and Mechanisms of Viral Membrane Fusion Proteins
- Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues
- Assessing ACE2 expression patterns in lung tissues in the pathogenesis of COVID-19
- High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa
- A pathological report of three COVID-19 cases by minimally invasive autopsies].pathological report of three COVID-19 cases by minimally invasive autopsies
Q19. Is a second wave of the pandemic likely to occur?
Last update: 15 June 2020
A second wave of infection refers to the phenomenon of re-emergence of infections in the population after they seem to have subsided. Currently, we do not have sufficient information to predict whether, and when, a second wave may occur. However, there are several possible reasons to expect a second wave:
1.Emergence of a new strain.
A new strain is a variant of the virus that differs in its biological properties, e.g. the ease of transmission, the way it is recognized by the immune system, or how it responds to medicines. A new strain could, therefore, manage to evade the immune system of even those who have recovered from the older virus strain, and cause re-infections. Although the COVID-19 virus is mutating slowly as compared to other coronaviruses, it has accumulated a few mutations. But the absence of experimental evidence for any biological differences between these isolates suggests that they most likely are the same strain. Hence, more studies are required to conclude if this could contribute to a second wave of infection.
2. Re-infection in a recovered population.
Recently, some cases of relapses of COVID-19 have been reported. Although a study in Rhesus macaques ruled out the possibility of re-infection in recovered animals exposed to the same strain, more studies are needed to confirm this effect in humans. Besides, the time taken to develop immunity and the duration for which it lasts remains to be determined. It is important to note that faulty testing could give a false impression of a relapse. False negatives or low viral load (that escapes detection with test kits) could lead to false conclusions of recovery, even though the infection may still be active. Such false negative cases could also contribute to a second wave of infection.
3.Community transmission after lockdown restrictions are lifted.
Lockdown can only slow down the spread of the virus, and should not be considered as a cure for COVID-19. Mathematical modelling already predicts a second wave of infection on relaxing interventions. Based on evidence for pre-symptomatic and asymptomatic transmission, a second wave is very likely to occur if people stop following preventive measures recommended by WHO, like physical distancing, wearing masks and washing hands regularly, after the lockdown is phased out.
References:
- Second wave
- WHO’s advice for public during COVID-19 pandemic
- Pre-symptomatic transmission
- Can someone be re-infected after they have recovered from Covid-19?
- Nurse in Chennai hospital succumbs to COVID-19, suspected case of relapse
- Reinfection could not occur in SARS-CoV-2 infected rhesus macaques.
- SARS-CoV2 mutation rate is much lower than other SARS-CoV 2002
- The Nextstrain
- Eight-strains-of-coronavirus-afflicting-the-world
- Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant
- First-wave COVID-19 transmissibility and severity in China outside Hubei after control measures, and second-wave scenario planning: a modelling impact assessment
- WHO ‘s advice for phased process of lifting lockdowns
- Video of flattening the curve
Q18. What is serological testing and how can it be used to detect COVID-19?
Last update: 15 June 2020
What do COVID19 serological tests detect?
Once a person starts showing COVID19 symptoms, over about one to two weeks, virus-specific antibodies begin to appear in their blood (Huang, Carreras et al., 2020). These are proteins produced by the immune system, that specifically bind to and neutralise viruses and help recovery. COVID19 serological tests (video: Serological tests 101) are designed to detect such neutralising or any other virus-specific antibodies in a blood sample (Vashist, 2020). As with pregnancy tests, serological tests are cheap and easy to do; and the results can be read out in minutes.
What do the test results mean?
A negative test result means that the blood sample does not have antibodies specific to the novel coronavirus. This could mean that the person has not been infected, or has been infected too recently for antibodies to be produced (Huang, Carreras et al., 2020, Vabret, Britton et al., 2020). A positive test result would mean that the person’s blood contains antibodies against the novel coronavirus. This could be because the person is currently infected and their immune system is fighting the virus; or because they have recovered and their immune system is still producing protective antibodies against the virus. For SARS-CoV1, a closely related virus, recovered patients continued to have detectable antibodies upto about a year after infection (Huang, Carreras et al., 2020, Vabret, Britton et al., 2020).
The immune system produces different kinds of antibodies at different times during and after infection. Tests may be designed to detect one or more of these antibody types. After an infection, the first antibodies to appear are IgM antibodies (within 1-2 weeks from infection for COVID19), followed by IgG antibodies (2-4 weeks, lasting upto a year after infection) (Huang, Carreras et al., 2020, Vabret, Britton et al., 2020). Tests that detect IgMs would detect infection earlier. IgG tests would indicate if a person has had the infection in the past, and would show a person’s long-term ability to fight COVID19.
Are serological tests reliable?
Antibodies against the novel coronavirus recognise and bind to specific molecules on the virus’ surface. Serological tests mimic these viral molecules to bind and detect antibodies in a blood sample. But different test products use different target molecules, and so they have different sensitivity (i.e. ability to detect small amounts of antibody) (Infectious Diseases Society of America guidelines). When a person is infected by other coronaviruses, e.g. those that cause common colds, they produce antibodies against them. But these antibodies might cross-react with the novel coronavirus target molecules in a serological test. If this happens, the test would give an incorrect positive result, without infection by the novel coronavirus (Infectious Diseases Society of America guidelines). All serological tests do not work in the same way (i.e. they are not standardised). The gold standard in COVID19 diagnosis remains PCR-based testing that detects viral genetic material (Esbin, Whitney et al., 2020). But these tests take about a day to run, require expensive laboratory equipment and supplies, and qualified technicians.
References:
Q17. What is herd immunity?
Last update: 15 June 2020
Herd immunity is the situation when a sufficiently high proportion of the population is immune to an infectious disease, slowing its spread in the population. The term ‘herd immunity’ is generally used in the context of immunity acquired via vaccines (see this animation). Sometimes, even if a vaccine is available and administered widely, some individuals remain unvaccinated. These could be infants, children, the elderly or people with certain medical conditions, depending on the disease/vaccine. They are not immune, yet have a much lower chance of getting infected, because the people they come in frequent contact with are immunized. They are thus protected by being part of a herd or a group. Herd immunity relies on immunized people to help break the chain of spread of infection.
Herd immunity is also possible if many people in a population become infected and then recover, so that they no longer transmit infection for the period during which they have active immunity. However, for a new infectious disease with adverse outcomes, it could be dangerous to depend on herd immunity for disease control before a vaccine is available.
More details about herd immunity
Herd immunity for an infectious disease is mainly decided by the reproductive number (R0) – the number of susceptible people that one infected person can directly infect in a population. R0 reflects ‘infectiousness’ of the disease (this video explains reproductive number). It decides the proportion (Pcrit) of the population that needs to be immune to make the entire population safe. The more infectious the disease, the higher the values of R0 and Pcrit. For example, measles is a highly infectious disease, with an R0 of about 12-18. As a result, 95% of the population has to be immunized (Pcrit = about 95) to provide herd immunity to the remaining 5%. On the other hand, seasonal influenza or ‘flu’ is less infectious (R0 is about 1-3) and herd immunity can be achieved by around 65% of the population being immune.
The exact conditions required for herd immunity are not easy to predict, because the reproductive number depends upon the population density and social structure. The protective effect of a herd can also get diluted if some people have weaker immunity against the pathogen, or if immunity decreases with time. Finally, If new strains of the virus/bacterium emerge, a large fraction of the population may become susceptible again.
COVID-19 and herd immunity
For the novel coronavirus, R0 is currently estimated to be about 3 (even higher in some countries). Thus, at least 70% of the population will have to be immune to the novel coronavirus, to ensure the safety of the remaining people. With no vaccine or cure available yet and a mortality rate of upto 3%, it would mean that a very large number of people will become severely sick with and/or die from COVID-19 before herd immunity is viable. Presently, following physical distancing and other precautions, and isolating patients and their close contacts, seems to be the only safe way to control the pandemic.
References:
- CDC glossary of terms for definition of herd immunity
- A video explaining herd immunity
- Paper on reproductive number and herd immunity for COVID-19
- A summary on herd immunity in some infectious diseases
- A summary of data on reproductive numbers for some infectious diseases.
- A popular science article on herd immunity basics and COVID-19
- A video explaining the reproductive number (R0)
- Another video on R0 and spread of an epidemic
Q16. What is convalescent plasma therapy, and can it be used to treat COVID-19?

Last updated: 1st May 2020
Convalescent Plasma therapy is a treatment strategy that has been used to combat diseases with no established cures. It has been used with some success to treat COVID-19. There are currently no proven drugs to treat COVID-19 (though early results of one study are positive for the drug remdesivir). . However when patients recover from the disease, it is because their immune systems successfully fought and cleared the virus from their bodies. Their immune cells produce antibodies that circulate in their blood finding and killing novel coronavirus. Could these antibodies produced by one recovered patient be used to fight the disease in another patient? Convalescent plasma therapy is a treatment strategy that does exactly that.
What is Convalescent Plasma therapy (CP)?
Antibodies are separated from blood donated by one recovered patient, and are given to another patient with a severe infection. These antibodies work in the second patient’s body to fight the virus for a few weeks. Eventually, the transfused antibodies would get depleted, but this buys the patient time to build up their own immune response (Marano et al., 2016).
Has CP helped fight other diseases in the past?
Convalescent plasma therapy has been used in the past to fight other new diseases such as Ebola (Winkler and Koepsell, 2015), and MERS-CoV (Arabi et al., 2016) which did not have approved medicines at the time. However, controlled clinical trials to test the efficacy of CP were not conducted.
Does CP help fight COVID-19?
In a few studies, patients with severe symptoms were administered CP therapy along with other treatments to manage symptoms (Duan et al., 2020), (Shen et al., 2020). There were no adverse reactions to the transfusions, and there was a marked reduction in disease severity over a period of days. However, these were not well controlled studies. There were no comparable patients who did not receive CP therapy; so it is not clear whether CP therapy was significantly beneficial. Nonetheless, the results so far are encouraging.
Is CP being used for treatment already?
The US Food and Drug Administration has not yet approved convalescent plasma as a treatment for COVID-19, but is recommending its use as an investigational treatment option. The US FDA, the Red Cross society and others are organizing blood donation drives for people who have tested positive and recovered from COVID-19. The Indian Council of Medical Research has also initiated clinical trials for CP to treat COVID-19, including two controlled studies.
References and more information:
- Convalescent plasma: new evidence for an old therapeutic tool?
- Effectiveness of convalescent plasma therapy in severe COVID-19 patients
- Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma
- The use of convalescent plasma to treat emerging infectious diseases: focus on Ebola virus disease.
- Feasibility of Using Convalescent Plasma Immunotherapy for MERS-CoV Infection, Saudi Arabia.
- Recommendations for Investigational COVID-19 Convalescent Plasma
- Donate COVID-19 Plasma
- Plasma Donations from Recovered COVID-19 Patients
- Call for Letter of Intent for Participation in: Therapeutic Plasma Exchange in COVID-19: Protocol for a Multi-center, Phase II, Open Label, Randomized Controlled Study
- ICMR plans plasma therapy clinical trials in 2 weeks to treat critical Covid-19 cases
- US FDA guidance on use as an investigational treatment.
- Article arguing the case for CP therapy for COVID-19.
- Guardian article about positive early results of Remdesivir study.
Q15. Can someone be reinfected after they have recovered from COVID-19?
Last updated: 21st April 2020.
Response: Definitive research about the progress of COVID-19 is still emerging. However, extrapolating from other coronavirus infections (SARS and MERS), and from current patterns of patient recovery, we eventually expect to see immunity in the population, even if it is short lived. As most individuals get infected and generate antibodies to fight the novel coronavirus, they are expected to develop immune memory, i.e. the capacity to produce antibodies against a specific strain of the virus. These antibodies neutralize the virus, thereby protecting us from further infection. Once a vaccine is developed against this specific strain, one can also become immune by getting vaccinated. Although multiple mutants of the virus exist worldwide, there is no evidence yet to suggest that they are serologically different strains (which antibodies against the earlier strain cannot recognize). Unless the virus mutates so much that it can evade the immune response in a recovered individual, a second re-infection by the same strain is unlikely. But scientists are still studying this phenomenon.
Currently, multiple genetic variants of the novel strain are being continuously reported [~10 groups/clades as of 24th Apr 2020, mistakenly called ‘different strains’ by some]. Since the mutations are accumulating slowly, if the disease can be contained, the likelihood of this strain developing into a serologically novel strain is low. Although most individuals who recover from COVID-19 are not expected to fall ill again with the same novel coronavirus strain, it is still unclear how long this immunity might last. A few reappearances of infection that are being reported could be either due to false-positive tests (i.e. errors in detection) or very low viral load that escaped prior detection, but became detectable later when testing was repeated.
References and more information:
- Herd Immunity – Immunisation Coalition
- Serotype
- Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic
- Nextstrain.org/ncov
- Eight strains of coronavirus afflicting the world
- Association of COVID-19 Infections in San Francisco in Early March 2020 with Travel to New York and Europe
- South Korea reports recovered coronavirus patients testing positive again
Q14. How does our immune response work and can we “boost” it to protect against COVID-19?

Last Updated: 21st April 2020
Response:
- (a) How does our immune response work? Immunity is like a country’s defense system. (i) Innate immunity is like maintaining a huge army (larger the better), to build a general defense against all types of attacks; (ii) Adaptive (antibody- and T-cell based) immunity relies on memory, remembering an earlier attack by a pathogen. When your body is invaded by the same pathogen again, it is able to quickly neutralize it via ‘surgical strikes’. However, since COVID-19 is caused by a new virus, if infected, we can rely only on innate immunity. If the viral assault is too strong, then this defense breaks down and disease sets in.
- (b) Is it possible, and advisable, to improve innate immunity? Research on animals shows that different parts of the immune response change in complicated ways with age, diet, and nutrition. Thus, we cannot say that a particular factor will enhance a specific part of the immune response against a specific pathogen. We need a lot more research to untangle these effects. Many people suggest that specific foods, herbs, or vitamin supplements can “boost immunity”. However, most of these claims are not supported by strong scientific evidence (i.e. large, randomized clinical trials). Overall good health is associated with improved ability to fight disease; but at present it is not clear that a specific aspect of “good health” is more important than others. Hence, physicians advise everyone to eat a healthy diet, exercise regularly and get enough sleep.
- (c) Is it advisable to boost innate immunity? It is unclear if “boosting immunity” would be a good idea. For instance, one of the hallmarks of COVID-19 in elderly patients is a “cytokine storm”, whereby an overactive immune response affects the body’s own cells and tissues, causing much more damage than the novel coronavirus.
- (d) How can adaptive immunity help against COVID-19? We develop immunity against viral diseases (such as influenza and SARS) by producing antibodies (adaptive immunity) against the specific virus. In fact, most vaccines artificially immunize populations by helping generate antibodies that help neutralize the specific virus. This immunity can be boosted naturally if there is re-infection by the same strain, or artificially by giving booster vaccine doses. Therefore, immunity against COVID-19 can only be boosted once a vaccine is developed or by using very expensive methods of passive immunisation (convalescent serum from recovered individuals).
References and more information:
- COVID-19: consider cytokine storm syndromes and immunosuppression
- A review of vaccine research and development: Human acute respiratory infections
- Subunit Vaccines Against Emerging Pathogenic Human Coronaviruses
- Booster vaccinations: can immunologic memory outpace disease pathogenesis?
- Master Question List for COVID-19 (caused by SARS-CoV-2)
- Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic
Q13. Can BCG vaccination protect me against COVID-19?

Last updated: 21st April 2020
Response: Vaccines are designed to work against specific pathogens (e.g. a species of bacteria or virus). The BCG vaccine trains our body to fight against Mycobacterium tuberculosis (the bacteria that cause tuberculosis). The key ingredient of the BCG vaccine is Mycobacterium bovis, a closely related bacterium that does not cause disease in humans, but that allows our body to respond to the tuberculosis bacterium, if it is encountered later in life.
Researchers have observed that children who receive BCG vaccination tend to have lower mortality from other infections (Shann 2013). However, many other factors that affect childhood mortality rate including economic and social can also affect this correlation; and it is unknown whether BCG vaccination in childhood can protect adults from infections.
Is there any evidence that BCG vaccination can protect against viral infections? In laboratory studies with cell cultures and mice, this does appear to be the case (see Moorlag et al 2019 and Covian et al 2019). One study also found that BCG vaccination in adults reduced viral load during yellow fever vaccination after a month (Arts et al 2017). The researchers showed that BCG vaccination improves non-specific immune responses to other pathogens through a phenomenon called “trained immunity”.
None of the above studies directly answers the question of whether BCG vaccination can prevent or rapidly cure an infection caused by the novel coronavirus. Two clinical trials to test this question are underway, and their results are awaited. At present the WHO does not recommend BCG vaccination as a strategy for dealing with COVID-19.
References and More Information:
Q12. Do chloroquine and hydroxychloroquine help in treating COVID-19?

Last Updated: 29 May 2020.
Response: Let us address this question step-by-step:
- What are chloroquine and hydroxychloroquine?
Chloroquine is a Food and Drug Administration (FDA)-approved antimalarial drug, known since 1934. Hydroxychloroquine, a derivative of chloroquine is also an antimalarial drug. Chloroquine or hydroxychloroquine are not yet approved for the treatment of COVID-19 and the WHO is conducting clinical trials to see if they would be effective.
- How do chloroquine and hydroxychloroquine act against the novel coronavirus?
After the outbreak of the Middle-East Respiratory Syndrome (MERS) in 2012, scientists showed that chloroquine could block that virus from infecting cells (MERS and SARS-COV2 are members of Betacoronavirus family). However, this was shown only on cells grown in the lab. The novel coronavirus (SARS-CoV2) fuses with the cells through a receptor, a protein called Angiotensin converting enzyme-2 (ACE2). The virus is swallowed into the cells through a process called endocytosis to form viral ‘endosomes’, which have a slightly acidic pH. Both chloroquine and hydroxychloroquine increase this endosomal pH, and that blocks the virus from taking over the host’s cells (Savarino et al., 2003). Chloroquine and hydroxychloroquine are also known to activate select pathways of the immune system which adds to their antiviral effect.
- Why are chloroquine and hydroxychloroquine not yet approved against COVID-19?
It is important to note that the clinical trials for chloroquine are currently ongoing. Few small-scale clinical trials done previously did not provide sufficient evidence on the safety and effectiveness of chloroquine/hydroxychloroquine (Gbinigie and Frie, 2020). A recent news article reported that a Brazilian trial had to be stopped as high doses of chloroquine resulted in irregular heart rates in patients. Before administering chloroquine, blood tests are necessary to test for anemia and precautions have to be taken to avoid cardiac, hepatic or renal complications (Cortegiani 2020). Data on the safety of chloroquine and hydroxychloroquine use is needed from many more high quality clinical trials before it can be clinically approved as a treatment against COVID-19.
- Latest situation, updated 29 May 2020
Since the original article on this subject, the US Federal Drug Administration and National Institutes of Health have both issued explicit advisories against using hydroxychloroquine or chloroquine to treat COVID19. This was after two very large studies (New York area study, cross-continental study) concluded that hydroxychloroquine or chloroquine, administered on their own or in combination with azithromycin, did not show any detectable improvement in chances of recovery from COVID19. In fact, patients who had been given these treatments had a somewhat higher chance of getting cardiac arrest, heart arrhythmias and death. A previous, smaller study (20 patients) had found a significantly positive impact of the drugs on COVID19 recovery. However, the methodology of this study has been widely criticised as being incorrect and the number of patients in the study was very small. Most health agencies do not recommend hydroxychloroquine to treat COVID19 because it does not improve chances of recovery, and in fact, increases risk of cardiac complications.A recently concluded, very large cross-continental study clearly shows increased mortality risk due to administering hydroxycholoroquine or chloroquine to COVID-19 patients.
As of the writing of this update, the Indian ministry of Health and Family Welfare has an advisory for the use of hydroxychloroquine as chemoprophylaxis for high-risk individuals. The rationale is that this is for disease prevention, not cure. According to the Indian Council for Medical Research, preliminary results show that hydroxychloroquine can help prevent COVID19. Note that the data are not yet publicly available and have not been scrutinized.
References and More information (original answer):
- Chloroquine for COVID-19 : Cutting through the hype
- Effects of chloroquine on viral infections: an old drug against today’s diseases
- Chloroquine is in clinical trials for efficacy against COVID-19
- Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro
- A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19
- Should chloroquine and hydroxychloroquine be used to treat COVID-19? A rapid review
- Small Chloroquine Study Halted Over Risk of Fatal Heart Complications
References (updated information, 29 May 2020):
JAMA study – related with cardiac arrest, no improvement in chances of recovery
Study reporting success, n of 20, widely criticised methodology
Cross-continental study showing increased mortality due to HCQ
Q11. Can children and young people get sick with coronavirus?

Last Updated: 17th April 2020.
Response: Yes. Data from WHO, as well as from China, shows that that the infection rate in children is between 1-2.4% (WHO-China Joint Mission Report). Children contract SARS-CoV-2 infection but are less likely to have severe symptoms. However, children can facilitate viral transmission by being asymptomatic carriers.
Severe Covid-19 symptoms are characterized by inflammation produced by chemicals made in the body leading to respiratory failure. The inflammatory responses vary in severity, and the extent is lower in children than in adults. This explains why children with Covid-19 do not readily show severe symptoms. 20% of the people hospitalized and 12% of ICU patients were 20-44 years old, CDC reports. In India 43% of confirmed novel coronavirus cases were between 20-39 years (India Today news report, April 3). Although lower numbers of younger people get sick, it is wrong to think that covid-19 affects only older people.
References and More information:
- Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19)
- The Coronavirus Disease 2019 (COVID-19) Outbreak in China—Summary of a China CDC Report
- Lancet Study
- COVID-19 in children and altered inflammatory responses
- CDC Study
- World Economic Forum report highlighting importance of CDC study
- Young Indians comprise more than half of confirmed Covid-19 cases
Q10. What makes the novel coronavirus (SARS-CoV2) more dangerous than Flu and SARS-CoV1 viruses?

Last Updated: 17th April 2020
Response: We are still learning about the novel coronavirus (SARS-CoV2) since it was discovered very recently. It differs from the flu and SARS-CoV1 viruses in many ways. First, it is much more contagious; i.e. each person infected with the new coronavirus can infect many more people. Second, symptoms take longer to develop; most infected people show either mild or no symptoms for up to 2 weeks. Since a person may not know they are infected, they unknowingly continue to infect many others. Third, the spike protein of the novel coronavirus binds much more strongly to human lung cells (works like a ‘hook’), increasing the chances of a successful infection. Thus, even though it is less likely to cause death, the novel coronavirus has the potential to cause much more damage to human populations.
- The reproductive number, R0 is the number of people that can catch the virus from an infected person. All else being equal, the higher this number, the more contagious the virus. While the flu (R0~1.3) and SARS-CoV1 (R0~1.17) viruses spreads to fewer people & hence more slowly, the novel coronavirus is highly contagious with an RO of 2.2 to 3.1 (each person is capable of infecting ~3 new people). This animation on Scientific American’s YouTube channel explains the R0 in more detail.
- The novel coronavirus spreads stealthily by causing only mild or asymptomatic infections in up to 80% of COVID-19 positive individuals (Maharashtra and China are notable examples). The viral load in sputum samples of asymptomatic carriers in China was high enough to be contagious.
- The death rate due to COVID-19 (1-2%) is 10-20 times higher than the Flu virus (0.1%), but is lower than previous SARS (11%) or MERS (35%) epidemics. However, since the disease spreads much more rapidly, it can cause more total deaths.
References and More information:
- Epidemiology Basics: Reproductive Number (R0)
- How does the new coronavirus compare with the flu?
- Model Parameters and Outbreak Control for SARS
- Coronavirus SARS-CoV-2 is spreading. Science uses this one number to figure out just how fast.
- Coronavirus(Covid-19) updates worldwide & India with state wise status
- Evolving Epidemiology and Impact of Non-pharmaceutical Interventions on the Outbreak of Coronavirus Disease 2019 in Wuhan, China (non-peer reviewed study)
- SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients
- Master Question List for COVID-19 (caused by SARS-CoV-2)
- How does the new coronavirus compare with the flu?
- COVID-19 basics
Q9. How long can the novel coronavirus survive in air and on various surfaces?
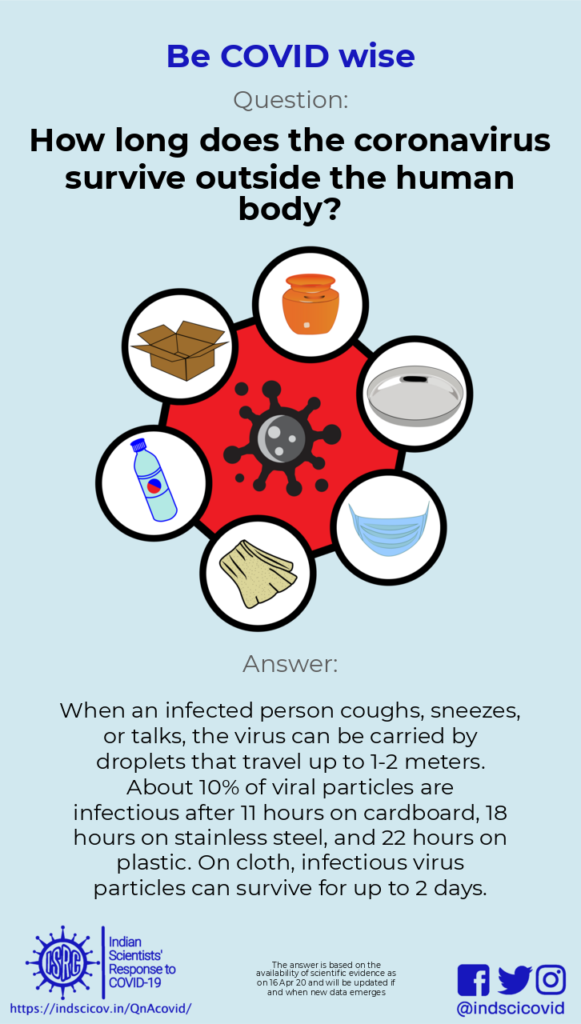
Last Updated: 17th April 2020
Response: Coughing, sneezing or even just talking generates droplets that can travel up to a couple of metres, before they settle on surfaces. Therefore, it is important to cover one’s mouth and nose when one sneezes or coughs. Researchers have shown that the novel coronavirus can transmit to another person through droplets in the air. When the coronavirus lands on surfaces, it stays infective for some time. The infectivity of the virus decreases in the air and on surfaces (including stainless steel, plastic, cloth, currency notes or facemasks). However, given the high infectivity of the virus, frequent hand washing using soap is highly recommended, and used masks should be disinfected or discarded. Simple homemade cloth masks must be washed thoroughly with soap and water and dried after each use (See the Do’s and Don’ts during COVID-19).
Researchers have studied the stability of the novel coronavirus on various surfaces and as droplets in air (see papers in NEJM and Journal of Hospital Infection). A recent paper in Lancet also reported the stability of the novel coronavirus on cloth. The infectivity of the virus decreases rapidly when it is in droplets and on surfaces. Researchers have reported that only 10% of the novel coronavirus particles retain their infectivity after 11 hours on cardboard, 18 hours on stainless steel or 22 hours on plastics. In the Lancet study, infective virus could not be detected on cloth two days after deposition; while on surgical masks (typically made of nonwoven plastic fibre), infective virus could be detected (at low concentrations) even after a week. Thus, it is important to handle masks very carefully, and thoroughly disinfect used masks (where appropriate) or dispose properly if the mask cannot be reused (see resources from WHO, MOHFW (GoI), Covid-gyan.in and CDC).
Those infected with the novel coronavirus (even if they do not show symptoms) can spread the virus through small droplets ejected when they cough, sneeze, spit or talk. Large droplets fall to the ground within 1-2 meters. This is why physical distancing is important. If infected people do not cover their nose and mouth when coughing or sneezing, small droplets can transport the long distances, even up to 7-8 meters. These droplets can deposit on surfaces, carrying the virus with them.
References and more information:
- NEJM paper on viability of new coronavirus on in air and on surfaces
- Persistence of novel coronavirus on surfaces
- Stability of novel coronavirus under different conditions
- Spreading of droplets on sneezing
- WHO – When and how to use masks
- Advisory on facemasks by Ministry of Health and Family Welfare, GoI
- Covid-Gyan advisory on homemade facemasks
- CDC:Decontamination and reuse of surgical facemasks and N95 respirators
- CDC: Homemade facemasks
Q8. What are the symptoms of COVID-19?

Last Updated: 17th April 2020.
Response: According to the WHO and the CDC, the most common symptoms of COVID-19 are fever, dry cough and shortness of breath along with fatigue. Some patients may have aches and pains, nasal congestion (sometimes loss of smell), runny nose, sore throat or even diarrhea. These symptoms are usually mild and begin gradually. It can take between 2-14 days to show symptoms of infection by the novel coronavirus.
Although some get infected but don’t develop any symptoms and don’t feel unwell, they must maintain physical distance away from family members. Most people (upto 80%) recover from the disease without special treatment. Some require hospitalization, and other conditions such as heart disease may lead to complications.
References and more information:
Q7. Will the summer temperature and humidity affect the novel coronavirus in India?

Last Updated: 17th April 2020.
Response: It is too early to predict how temperature or humidity will affect the novel coronavirus. While cold and flu spread more easily in winter than in summer, not enough is known yet about the novel coronavirus. One preliminary study suggested that the spread might reduce in summer. This must be interpreted with caution, since many people have contracted COVID-19 in other tropical countries like Philippines, Malaysia, and Singapore despite high temperature and humidity.
Besides temperature and humidity, human behaviour (physical distancing, handwashing) and other factors cumulatively determine the seasonal spread of other coronavirus infections. One lab study suggests that the novel coronavirus can survive for more than seven days at 22℃ and relative humidity of 65%, but is progressively inactivated within 30 min at 55℃ or within 5 min at 70℃ (much higher than even the hottest summer temperature). In the current COVID-19 outbreak, data from a preliminary study seemed to suggest that infection was spreading slightly faster in cities with colder temperature and lower humidity. Although another study (still under scrutiny) also predicts that contagious spread might decrease moderately in summer, the current spread of the novel coronavirus (Worldometer) indicates that it is uncertain whether higher temperatures or humidity will stop the virus. The Indian Ministry of Health and Family Welfare, WHO and CDC, all suggest that citizens follow the recommended precautions, irrespective of the weather.
References and more information:
- Seasonality of Respiratory Viral Infections | Annual Review of Virology
- Stability of SARS-CoV-2 in different environmental conditions
- Potential Factors Influencing Repeated SARS Outbreaks in China
- High Temperature and High Humidity Reduce the Transmission of COVID-19
- https://www.worldometers.info/coronavirus/#countries
- Coronavirus: Will summer make a difference?
- https://www.cdc.gov/coronavirus/2019-ncov/faq.html
Q6. How do droplets spread Covid-19?
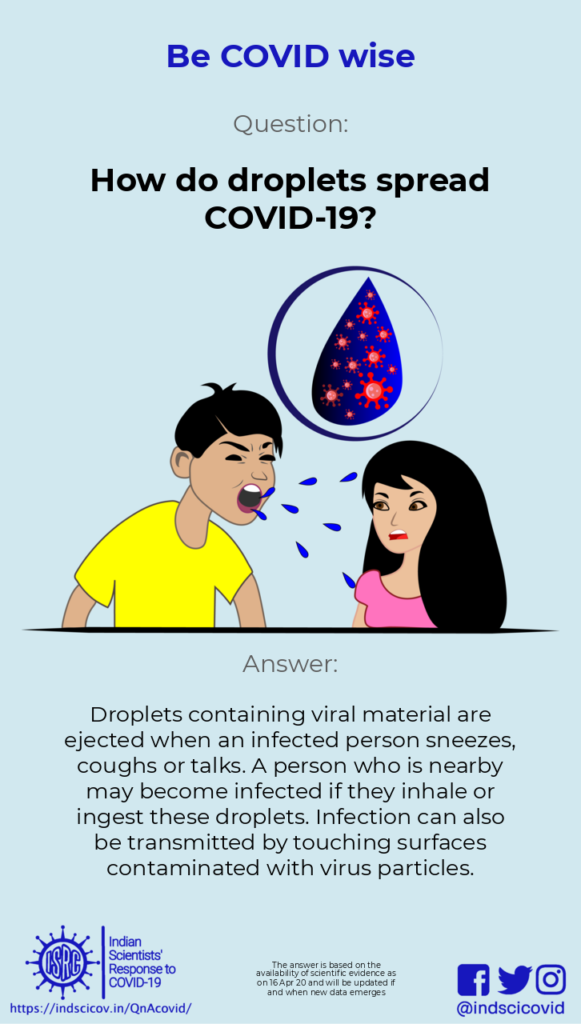
Last Updated: 17th April 2020.
Response: When one sneezes, coughs or just speaks, droplets (defined as greater than 5-10 microns in size) and aerosols (less than 5 microns in size) are ejected from their mouth or nose. Droplets are relatively heavy and settle quickly. If the virus in these droplets comes in contact with the nose or mouth of another person (within 1-2 meters from an infected person), that person gets infected. This is a major route of transmission of the novel coronavirus. Therefore, it is important that everyone use masks to avoid spreading or inhaling droplets. Wearing a mask also reduces the chances of contact-mediated transmission, as you are less likely to touch your face.
If droplets from an infected person land on a surface, the virus is deposited on it. The virus is rapidly deactivated on surfaces, but on stainless steel or plastic, it can stay infective for several days. After that, the viral particles may become less infective.
The US CDC and European CDC state that it is possible for a person to be infected by touching a surface that has infective viral particles on it. The novel coronavirus can survive on surfaces, but the concentration of infectious virus particles decreases with time. Also, a minimum number of viable viral particles are required for a person to get infected. Though touching surfaces is not considered to be the main route of transmission, it is advisable to avoid unnecessary contact with outside surfaces, and wash hands frequently with soap.
References and more information:
Q5. How long does it take before one shows symptoms of Covid-19?

Last Updated: 17th April 2020.
Response: WHO estimates that it takes about 5 days to show symptoms of Covid-19, if one is infected. However, this time called the “incubation period” could be anywhere from a day to about 2 weeks. Note that the novel coronavirus can also be transmitted by those who only show some of the symptoms, such as coughing.
People are thought to be most infectious within a few days of onset of symptoms. But some people infected with coronavirus never show any symptoms but can still infect others in what is called asymptomatic transmission. People might also be infectious before they start showing symptoms (called pre-symptomatic transmission).
The time from when an infected person shows symptoms to when the next person they infect shows symptoms, is about 4 days, (as shown in a recent study). Since this time is less than the average time to show symptoms (5 days), physical distancing is critical.
Results of a modeling study show that there was substantial variation in novel coronavirus transmission over time, and a decline in transmission in Wuhan, China around the time that control measures were introduced.
References and more information:
Q4. How does physical distancing help reduce the spread of novel coronavirus?
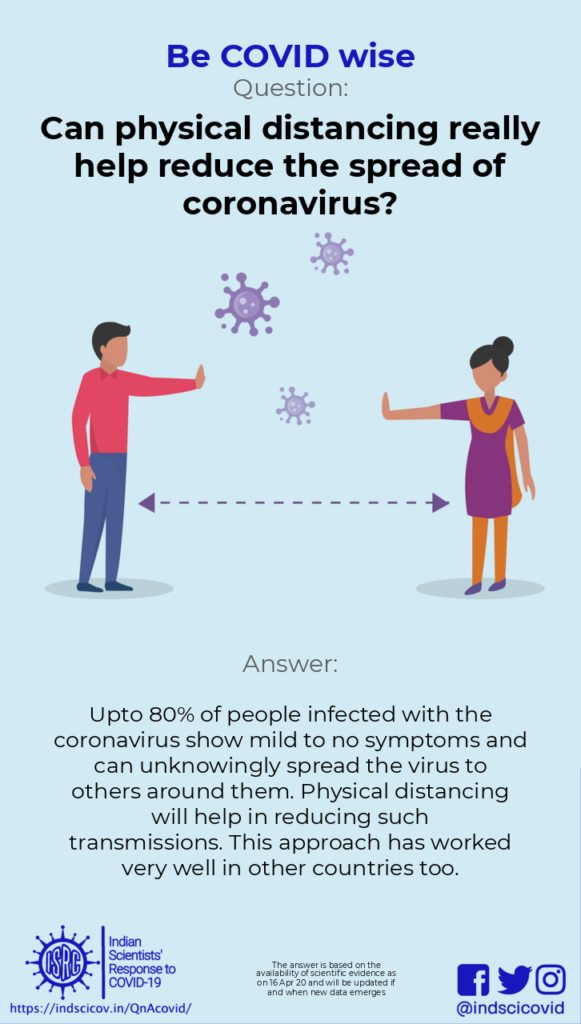
Last updated: 11th April 2020.
Response: Upto 80% of the people infected with the novel coronavirus show mild/no symptoms and can unknowingly spread the virus via their respiratory droplets. Physical distancing greatly helps to reduce such transmissions.
To ‘maintain physical distancing’ means people must keep a distance of at least 6 feet between them. The novel coronavirus is transmitted through respiratory droplets (generated while coughing or sneezing), or by contact with a contaminated surface (where droplets settle). As seen in various countries, anywhere between 25-80% of infected people may show mild symptoms or none at all (these are called asymptomatic transmissions). People with clear symptoms of COVID-19 are likely to be isolated and quarantined. However, many asymptomatic people might spread the virus unknowingly.
Many studies have reported a decline in the number of new cases after introducing strict physical distancing and isolation policies. China, which was the epicentre of the pandemic, also imposed strict isolation policies and at the end of March 2020 it appears to have decreased the spread of coronavirus (Cyranoski, 2020).
Physical distancing; washing hands frequently and thoroughly; other personal hygiene practices; seeking medical intervention when needed, are ALL necessary to reduce the spread of the novel coronavirus.
References and More information:
Q3. I have heard that there are many types of coronaviruses. Can they not infect humans?

Last updated: 11th April 2020.
Response: Many different types of coronavirus do infect animals (e.g. bats, pigs, camels). As they evolve, coronavirus can sometimes jump from these animals and begin to infect humans (called a “spillover”). Currently, we know of seven different coronavirus that can infect humans, including some that cause common cold. Some coronavirus infections can be fatal.
The genetic material of the novel coronavirus (RNA) is closely related to the types of coronavirus that infect bats and pangolins, suggesting that it may have jumped to humans from one of these (or related) host species.
References and More information:
Q2.Why does it take a long time to make a vaccine against the novel coronavirus? Can the process be made faster?

Last updated: 15th May 2020.
Response: A vaccine ‘imitates’ a particular pathogen (such as a virus), without harming the host. It consists of parts of a pathogen (e.g. key proteins) or a weakened whole pathogen. Vaccines prepare our immune system to fight against the pathogen before the body is actually infected. This document from the CDC, and this video, explain how vaccines work.
From designing to mass production, making vaccines takes a long time because it is important to ensure that it works and is safe for humans. Vaccines are first tested in preclinical stages, i.e., on tissue cultures and lab animals, like mice. This is done to check if the designed vaccine elicits an immune response; and this can take upto 2 years. This is followed by clinical trials in 4 stages, where the vaccine is tested on human volunteers. The clinical trials ensure that the vaccine is safe, efficient, and generates the expected immune response. More details about vaccine development can be found here.
In the case of the novel coronavirus, very little was known at the start of the outbreak. The genome sequence of the virus was released in Jan 2020. This made it possible for scientists to target certain proteins and genes for vaccine development. E.g., the novel coronavirus’ spike glycoprotein is reported to elicit an immune response, making it a potential vaccine target (Walls et al., 2020). Currently, there are at least 42 candidate vaccines under development, that include inactivated whole viruses, protein subunits, and viral genetic material. Each of these is under various stages of preclinical studies or clinical trials (Chen et al., 2020, WHO draft landscape).
Typically, the first two stages of clinical trials take upto a year. The third and fourth phases may take more than 3 years, depending upon the duration for which the vaccine’s safety and long-term effects are to be tested. Although the COVID-19 vaccines are being fast-tracked into clinical trials, it may still take a year for a vaccine to be commercially available.
Update 14/5/2020
Within a month since this article was written, the number of vaccines in development around the world has more than doubled to over 100 (Milken Institute Vaccine Tracker, WHO draft landscape, Biorender Vaccine tracker; Graphical guide to coronavirus vaccines). Of these, more than 10 have progressed to the stage of clinical trials with human volunteers. Typically, the following stages would take many months to years to complete, but given that no other treatments/vaccines exist for COVID-19, the process has been fast-tracked. One of the front-runners is the ChAdOX1 vaccine being developed at Oxford University. This vaccine candidate showed positive results in a primate study; clinical trials in humans have begun (Oxford Vaccine Group) and are expected to be completed by May 2021 (US clinical trials registry). The Serum Institute of India has started ramping up production of this candidate vaccine even before it has cleared clinical trials (NY Times article). There has been rapid progress and encouraging early results in COVID19 vaccine development, but we are still many months away from widespread availability of a proven vaccine.
References and More information:
- Understanding how vaccines work
- Vaccine Development, Testing, and Regulation
- The SARS-CoV-2 Vaccine Pipeline: an Overview by Chen et al 2020
- DRAFT landscape of COVID-19 candidate vaccines – 20 March 2020
- Structure, function and antigenicity of the SARS-CoV-2 spike glycoprotein by Walls et al 2020
- Coronavirus | Vaccine was rapidly synthesised as novel coronavirus sequence was available, says virologist Gagandeep Kang
Q1. What exactly is a testing kit? Why is it difficult to make one for COVID-19, and why do the results take time?

Last Updated: 11th April 2020.
Response: Currently, the most widely used test for COVID-19 is based on detecting the virus’ genetic material (RNA). The main problem is that patient samples have very little viral RNA but a lot of human genetic material (DNA). This problem is overcome using a method called real time RT-PCR (Reverse Transcription – Polymerase Chain Reaction), to increase the amount of viral genetic material and make it easier to detect. This infographic summarizes how the test works.
A sample is taken from the back of a person’s nose and throat area. Testing kits contain chemicals that first separate genetic material (RNA and DNA) in the sample, and then copy all the RNA into DNA (which makes the next step easier). After making thousands of copies of this viral DNA (ignoring human DNA), it is relatively easy to detect. The test is positive if viral DNA is found at the last step.
Although RT-PCR is commonly used for research, only a few companies manufacture kit components. The kits need to pass very high standards of quality control before they can be reliably used for patient samples. The RT-PCR based test can be completed in a few hours, but in India, samples can be tested only in an authorized testing laboratory.
A second testing method detects antiviral antibodies produced as part of the body’s immune response to an infection. Antibodies are proteins that specifically bind to a pathogen (virus) and help to inactivate it. Antibody tests require a blood sample, and can produce results in less than an hour. However, they can only detect a past (recent) infection; they cannot detect a current infection.
References and More information: